

This is because we know that the substance has zero entropy as a perfect crystal at 0 K there is no comparable zero for enthalpy. In cases for which we want to calculate the entropy change S for a given chemical reaction, we follow a procedure similar to that used in calculation of H. The reason is that the entropies listed are absolute, rather than relative to some arbitrary standard like enthalpy. Note that there are values listed for elements, unlike DH fº values for elements. The Thermodynamics Table lists the entropies of some substances at 25 ✬. The entropy change formula for chemical reactions is Sreaction. Continue this process until you reach the temperature for which you want to know the entropy of a substance (25 ✬ is a common temperature for reporting the entropy of a substance). The change in entropy measures the difference between the entropy of products and reactants. Then you can use equation (1) to calculate the entropy changes. Even though equation (1) only works when the temperature is constant, it is approximately correct when the temperature change is small. Now start introducing small amounts of heat and measuring the temperature change. Dr Biró and colleagues have made strides towards generalizing Boltzmann’s original formula.

As such, the team’s master equations could model the changes in entropy of real systems over time. Since there is no disorder in this state, the entropy can be defined as zero. Stable nonlinear master equation models describe a change of probabilities always towards a no-more-changing, stationary distribution. Imagine cooling the substance to absolute zero and forming a perfect crystal (no holes, all the atoms in their exact place in the crystal lattice). The absolute entropy of any substance can be calculated using equation (1) in the following way. At absolute 0 (0 K), all atomic motion ceases and the disorder in a substance is zero.
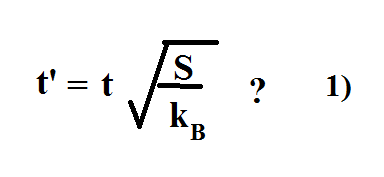
On this scale, zero is the theoretically lowest possible temperature that any substance can reach. The temperature in this equation must be measured on the absolute, or Kelvin temperature scale. Using this equation it is possible to measure entropy changes using a calorimeter. Where S represents entropy, DS represents the change in entropy, q represents heat transfer, and T is the temperature. ItmaybehelpfultoseethatifweuseGibbsentropyde nitionthatentropydoesinfactincrease duringdi usion.Forsimplicityconsideradi usinggasin1dimension,withnumberdensityn(x t). One useful way of measuring entropy is by the following equation:


 0 kommentar(er)
0 kommentar(er)
